Essential plant nutrients are elements that plants need for proper growth. Sixteen elements are considered essential nutrients for plants. These are carbon (C), oxygen (O), hydrogen (H), nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), boron (B), molybdenum (Mo) and chlorine (Cl).
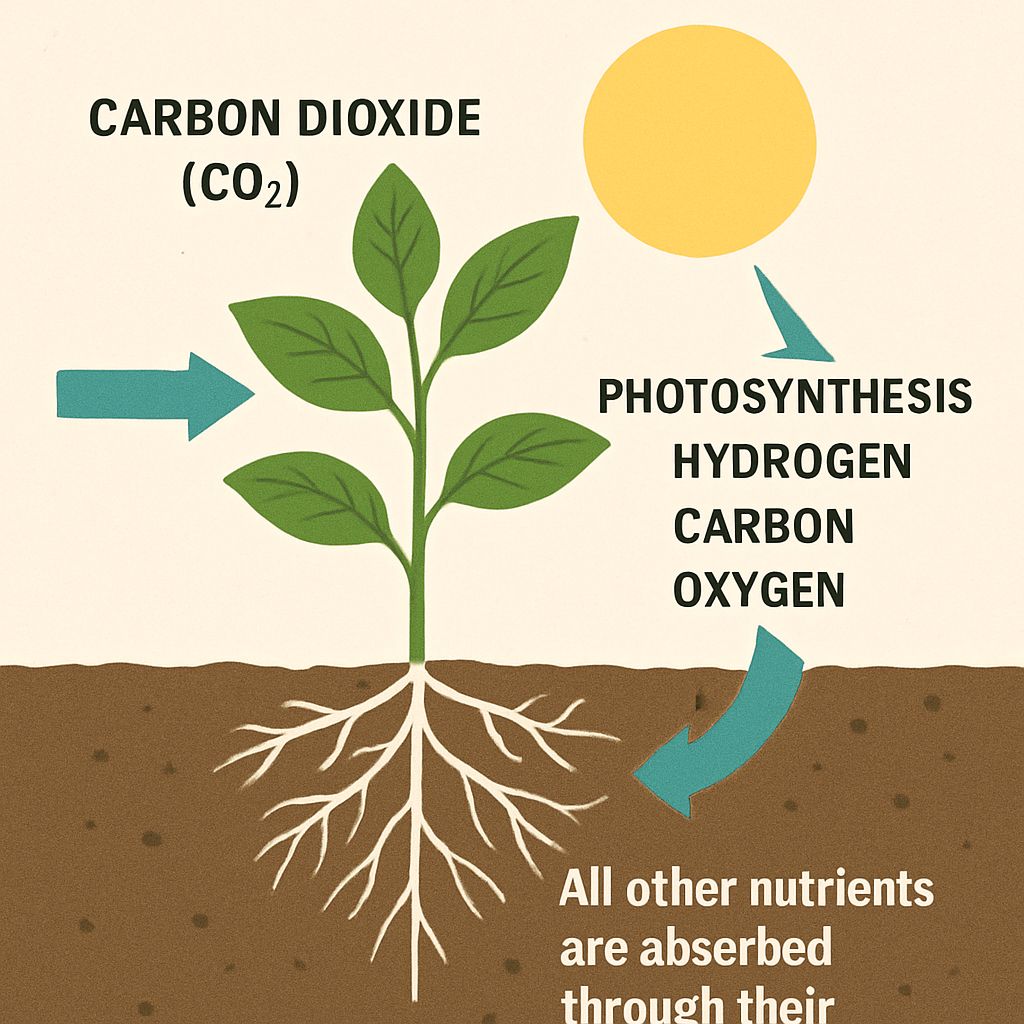
Plants absorb carbon and oxygen from the air through their leaves, as carbon dioxide (CO2). In the photosynthesis process, they transform carbon dioxide and water into hydrogen, carbon and oxygen. All other nutrients are absorbed through their root system.
Plants from the Legumes family can use atmospheric nitrogen. They form a symbiotic relationship with specific bacteria that convert atmospheric nitrogen into ammonia and then into ammonium, which the plant can absorb. This process is called ‘nitrogen fixation’.
NUTRIENT CATEGORIES
The essential plant nutrients can be categorized as macronutrients, secondary nutrients and micronutrients. This classification is based on the relative requirement by the plant.
Macronutrients are required in relatively large quantities. Secondary nutrients are required is lesser amounts and micronutrients are required in very small amounts. This does not imply that micronutrients are less important to the plant. A deficiency of one micronutrient can limit the growth of the crop to the same extent as a deficiency in macronutrients do.
Macronutrients include nitrogen, phosphorus, potassium, carbon, hydrogen, oxygen
Secondary nutrients – calcium, magnesium, sulfur
Micronutrients – boron, iron, manganese, zinc, copper, molybdenum, chlorine
Common average nutrient requirements of crops are in the following ranges:
| Macronutrients | Typical daily uptake | Secondary nutrients | Typical daily uptake | Micronutrients | Typical daily uptake |
|---|
| Nitrogen (N) | 1.5-4 kg/ha (1.3-3.5 lbs/acre) | Calcium (Ca) | 0.5-1.5 kg/ha (0.45-1.3 lbs/acre) | Iron (Fe) | 20-50 g/ha |
| Phosphorus (as P2O5) | 0.3-0.7 kg/ha (0.25-0.6 lbs/acre) | Magnesium (Mg) | 0.2-0.5 kg/ha (0.15-0.5 lbs/acre) | Manganese (Mn) | 5-20 g/ha |
| Potassium (as K2O) | 1.5-5 kg/ha (1.3-4.4 lbs/acre) | Sulfur (SO42-) | 0.2-0.5 kg/ha (0.2-0.5 lbs/acre) | Zinc (Zn) | 5-10 g/ha |
|
|
|
| Copper (Cu) | 2-8 g/ha |
NUTRIENT UPTAKE BY PLANTS
Plants can absorb specific ionic forms of the nutrients, as described in the table below. In that respect, nitrogen is unique, as it can be absorbed either as an anion (NO3–) or a cation (NH4+). The two nitrogen forms are very different in their metabolism within the plant and in their effect on the root system environment.
| Nutrient | Form/s in which it is absorbed by plants | Nutrient form name |
|---|
| Nitrogen (N) | NO3–
NH4+ | Nitrate
Ammonium |
| Phosphorus (P) | H2PO4–
HPO42- | Dihydrogen phosphate Hydrogen phosphate |
| Potassium (K) | K+ | Potassium |
| Calcium (Ca) | Ca2+ | Calcium |
| Magnesium (Mg) | Mg2+ | Magnesium |
| Sulfur (S) | SO42- | Sulphate |
| Boron (B) | H3BO3 | Boric acid |
| Iron (Fe) | Fe2+
Fe3+ | Ferric
Ferrous |
| Manganese (Mn) | Mn2+ | Manganese |
| Zinc (Zn) | Zn2+ | Zinc |
| Copper (Cu) | Cu2+ | Cupric ion |
| Molybdenum (Mo) | MoO22+ | Molybdate |
UPTAKE BY GROWTH STAGES
Plants absorb nutrients at different rates throughout their development cycle. Generally, the uptake rate is lower at the beginning of the growth cycle, increases during fruit development, and drops just before harvest. Furthermore, uptake rates of individual nutrients vary along the growth cycle. For example, plants require more nitrogen during the establishment and vegetative growth stages, while potassium is required in greater amounts during the fruit set period.
NUTRIENT AVAILABILITY
Not all nutrients that are present in soil are available for plants. In fact, most of the nutrients in soil are locked up in minerals or in organic matter and only a small fraction becomes available for plant uptake.
Plant roots can absorb nutrients only from aqueous solutions. Therefore, in order for a plant nutrient to become available to the plant, it must first be “delivered” into the soil solution.
The processes that are responsible for that are:
Dissolution of soil minerals.
Chemical equilibrium between nutrients that are adsorbed to soil particles and the soil solution (exchangeable cations).
Mineralization
Soil pH affects all of these processes and therefore plays a major role in nutrient availability. Additional factors that affect nutrient availability include specific bacteria that mineralize nitrogen and phosphorus, the balance between nutrients in the soil and more.

 English
English Español
Español
